Important guidelines for PPP registration
-
Following directive regulates the registration of Plant Protection Products:
-
Regulation (EC) 1107/2009
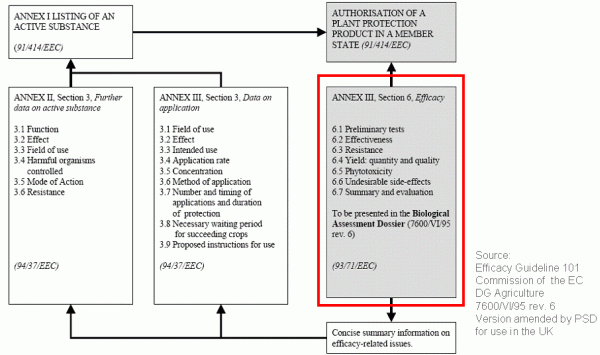
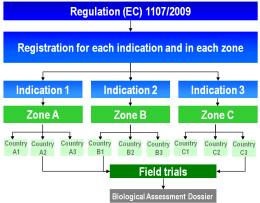
Under Regulation (EC) 1107/2009 the definition of three geographical zones aims at harmonising, streamlining and accelerating the registration of plant protection products. Within a zone, the authorisation of a product (an indication = the combination of crop, pest/disease and plant protection product) has to be applied for in only one member state which has been designated as zonal rapporteur.
-
The necessary field trials must be conducted at different sites within one zone or one country over a two to three years period. However, the risk does exist that these trials may not be accepted by the authorities and must be repeated or extended. In certain cases, national registration authorities may reject the otherwise mandatory zonal mutual recognition. In these cases, field trials have to be repeated in the member states concerned within the zone.
Both, additional field trials on the one hand and a delayed market launch of the product on the other hand, lead to increased expenses.
-
Please find further information about the registration of Plant Protection Products on the following websites:
- Federal Research Center for cultivated plants - Julius Kühn-Institut
- Federal Office of Consumer Protection and Food Savety
-
Related Links
Please find information about EU Regulation EC 1107/2009, and particularly about the issues:
- Zonal approach and
- Mutual recognition